Table of Contents
- Definition of Decomposition
- Types of Decomposition
- Stages of Decomposition
- Decomposition in an Ecosystem
- Factors That Affect The Rate of Decomposition
- Decomposing Body
- Types of Chemical Reactions in Decomposition
Definition of Decomposition

Decomposition can be defined as a metabolic process through which organic bodies, structures, and substances that have recently died are acted upon by internal and external organic and non-organic substances that decay and break down the organic bodies into simpler forms of organic and inorganic matter and energy.
In the case of vertebrates, this complex process begins immediately after death and is triggered by two key processes. Autolysis (the digestion of cells through the action of their own enzymes) and putrefaction (the fifth stage of death in which the anaerobic decay of organic matter is perpetrated by bacteria and fungi).
Types of Decomposition
There are primarily two main types of decomposition under which all other related aspects fall. Biotic decomposition and abiotic decomposition.
Biotic Decomposition

Biotic decomposition is the process through which dead organic matter is recycled back into the ecosystem by being broken down, decomposed, or degraded into energy and smaller simpler organic and inorganic matter.
The process is generally carried out by living organisms from the community of microbiota that are indigenous to that particular environment. The process is aided by some environmental abiotic influences. This includes chemical reactions which can also take place in the process of biotic decay. However, the process will not occur without the activities of indigenous microbiota.
Microbiota is a collective term that represents ecological communities of commensallic (long term association between different species of organisms where one species benefits and the other is unaffected), pathogenic (potentially inflicting disease or harm), and symbiotic (any type of close biological association between different species of organisms) microorganisms that are abundant in the ecosystem. They can be found on all multicellular organisms including plants and animals.
The microbiota community includes bacteria, archaea, viruses, protists, and fungi and to a large extent, are responsible for the process of decomposition.
Categories of Biotic Decomposition
There are two categories of biotic decomposition that are classified based on the conditions under which the decomposers metabolize organic material into energy, micronutrients, organic, and inorganic matter.
- Aerobic Decomposition
- Anaerobic Decomposition
Aerobic Decomposition

Aerobic decomposition is the metabolic process through which organic matter is acted upon, broken down, and disintegrated into simpler forms of organic and inorganic matter and energy by aerobic microorganisms in the presence of oxygen.
Anaerobic Decomposition

Anaerobic decomposition usually termed anaerobic digestion, is the metabolic process through which organic matter is acted upon, broken down, and disintegrated into simpler forms of organic digestate (a nutrient-rich substance produced by anaerobic digestion) and inorganic matter and energy like biogas by digestive decomposition carried out by a complex variety of symbiotic anaerobic microorganisms in the absence of oxygen.
The process of anaerobic decomposition where symbiotic microorganisms act upon and convert organic substances into nutrients, organic cell by-products, refractory (substances that are highly resistant to decomposition) substances, simple salts, and pungent biogases is essentially the same process as putrefaction.
Factors that affect Biotic Decomposition
The main factors that affect biotic decomposition are as follows.
Freeze-Thaw cycles
Periodic and temporarily frozen soil, ground moisture, frozen water bodies, and permafrost (soil, rock, or sediment that freezes for over two years) constitute the main elements responsible for freeze-thaw cycles (reoccurring seasonal changes involving winter freezing or icing up, and melting/thawing ice during spring).
This cycle plays an important role in the distribution and balance of soil nutrients as well as the overall polar ecology (the interaction between plants and animals in the Arctic or Antarctic regions).
This is due to the effects seasonal freezing and thawing have on the soil by delaying its summer warming and winter cooling of the surface. Thawing can be simply defined as the process of a previously frozen substance warming up and becoming unfrozen. This is typically characterized by melting ice.
It also enhances soil hydrology (the scientific discipline that analyses the composition, distribution, and circulation of the earth’s water) by temporarily hindering soil drainage (the downstream removal of moisture from the ground); and accumulating a high soil moisture content in the active layer (upper soil layer that is subjected to seasonal freezing and thawing).
The frozen surfaces hinder virtually all microbial action and preserve organic material in a state of suspended animation (temporary slowing or stopping of biological activity including locomotion). Leaching plays a more important role in moving nutrients around after thawing when freezing temperatures have killed many microorganisms in the soil.
This can be particularly important in spring when the soil thaws releasing nutrients that had been trapped and dormant in the freezing temperatures. This shows that frozen soil also preserves soil nutrients that might have otherwise been washed away or used up earlier that are released and redistributed when the soil thaws.
Wet/Dry Soil
Decomposition rates are low under very wet or very dry conditions in an ecosystem. They are generally higher in wet or moist conditions with a sufficient amount of oxygen.
Very wet soils tend to be deficient in oxygen levels especially in the wetlands which slow microbial growth. Decomposition slows in dry soils, but bacteria will generally continue to grow even though at a much slower rate, even when conditions are too dry to support plant growth.
Taphonomy
Taphonomy is the scientific study of the processes of how organisms and organic matter through decomposition and a systematic series of transformations, transition from the biosphere (or ecosphere is the collection of all the ecosystems that exist on Earth) to the lithosphere (the solid outer layer of the Earth).
The study analyses the key processes that cause the transition especially the stages of decomposition and how organisms transition from autolysis at the early stage of death to fossilization (the process of the hard remains or imprint of animals or plants being preserved as a cast, calcified form, or impressed in hard medium).
This usually occurs long after skeletonization (complete decay of soft tissues of an animal leaving the skeleton) in the final stages of decomposition.
Taphonomy is generally divided into two parts or phases of study.
The first part examines the processes that occur from the death of the organism to the period it is either buried or being integrated or incorporated into the soil and surroundings after the conclusion of the process of decay. This part is referred to as the biostratinomy phase and involves biotic processes.
The second part is the period and processes that occur just after the first phase which is the period after the burial or integration into the ground after the conclusion of the process of decay.
This part is called the diagenesis stage and involves mostly abiotic processes like the natural exertion of physical and sub-terranean pressure and temperature, chemical changes in sediments from water/rock interactions, and compaction, but often also involve biotic activity like microbial action.
There are five main identifiable stages of taphonomy namely:
- Disarticulation
- Dispersal
- Accumulation
- Fossilization
- Mechanical alteration
Disarticulation
Disarticulation is the detachment and separation of limbs and bones from tendons and joints effectively removing them from the body. It occurs partly due to decay and partly from exposure to atmospheric and climatic exertions.
The action of scavengers can also contribute to this condition. Disarticulation and dispersal are the only two stages of taphonomy that are partly caused by biotic factors.
Dispersal
Dispersal in taphonomy is the separation of various pieces of an organism with bits of the body strewn randomly in different directions over areas that are of different distances from the parent body.
This may be caused by natural events like the effects of climate in the instances of flooding, wind, or earth-moving events like earthquakes, landslides, or erosion. Dispersal can also be caused by the actions of scavengers or human/animal activity.
Accumulation
Accumulation refers to the gathering and build-up of organic and inorganic material in a common area which is often a result of the movement of groundwater, or run-off water from rain but can also be aided by factors like earthquakes, landslides, wind, and gravity.
Fossilization
The permeating (pa) of mineral-rich groundwater and liquefied minerals into organic materials and cavities in dead organic matter which calcifies (harden by deposition of or conversion into calcium carbonate or some other insoluble calcium compounds) or otherwise solidifies within these materials taking their shapes and forms is termed as fossilization.
The remains may be solidified permanently preserved or imprinted forming the fossils. Generally remains are only considered fossilized after they have been existing for up to ten thousand years or more.
Although these stages are successive, they are by no means strictly so throughout the process due to some processes like chemical reactions due to the action of bacteria and in some instances fragmentation occurring at virtually all or some of the stages.
Mechanical Alteration
Mechanical alteration is the stage where the physical attributes of organic or inorganic matter are changed in some physical form or the other as a result of chemical and mechanical or physical exertions such as heat, atmospheric pressure, acidic precipitation, freeze-thaw cycles, frictional abrasions from transportation, or being moved, pressure from burial and compaction.
Stages of Decomposition

There are basically five stages in the process of decay or decomposition of vertebrates. It begins with the initial breakdown that occurs at the fresh stage just after death when the body begins to cool down to match the surrounding temperature.
Starved of blood, capillary action, and oxygen flow, tissues and muscles become stiff, rigid, and drained of blood as it gravitates towards the lower parts of the body and pools around the resting surface.
Bacteria contained in the intestines begin to act on its inner walls as cells begin to lose their structural integrity releasing enzymes that start the process of autolysis (or self-digestion is the dissolution of the cell by the actions of its own enzymes or the digestion of an enzyme by a molecule of the same enzyme).
Autolysis and putrefaction (the advanced stage of decay) which begins at the second stage of decomposition are the two stages of decay that involve the chemical process.
They are also directly linked to the stages of death (autolysis, pallor mortis, algor mortis, rigor mortis, livor mortis, putrefaction).
The five stages of decay are as follows:
- Fresh
- Bloat/putrefaction
- Active decay
- Advanced decay
- Dry remains
Fresh
The fresh stage of decomposition in vertebrates begins as soon as the heart stops beating. In the following moments after death, pallor mortis and algor mortis set in, and the body temperature begins to rise or drop to match the surrounding temperature.
Autolysis
The stopping of the heart ceases all circulation in the body which leads to a lack of oxygenated blood and a failure to remove carbon dioxide from the tissues.
This results in a drastic drop in pH and the high energy molecules that are necessary for the maintenance of cell integrity and homeostasis (a self-regulating process through which an organism maintains its functional stability while adjusting to conditions to best suit its survival).
These conditions also lead to the rupture or disintegration of membranes and the release of cellular enzymes that begin to break down or dissolve cells and tissue. This process is termed autolysis.
The fresh stage of decay does not typically display obvious external signs other than the pale appearance and slight blistering in some cases.
Aerobic microbes (organisms that survive and thrive in oxygenated surroundings ) and cellular metabolism (chemical reactions that occur in living organisms) that are naturally present in the gastrointestinal (the entire nutritional passage from the mouth to the anus including the organs of the digestive system) and respiratory tracts use up the quickly diminishing volume of oxygen in the system.
The resulting deficiency of oxygen promotes the generation and multiplication of anaerobic (microorganisms that don’t require molecular oxygen to live and grow) organisms that rapidly multiply.
These anaerobic organisms consume the body’s carbohydrates, lipids, and proteins in a process that produces compounds like ammonia, lactic acid, methane fumes, propionic acid, and hydrogen sulfide.
This process of microbial digestive and multiplying action that causes the accumulation of anaerobically generated putrid gases and materials is termed putrefaction which begins to occur at the bloat stage of decomposition.
Bloat/Putrefaction
Bloat is the second stage in the process of decomposition and is usually the first externally visible sign of ongoing microbial proliferation (microscopic bacterial growth).
The anaerobic metabolic processes intensify and are by this point consuming much of the internal soft tissues, multiplying and producing large volumes of noxious gases including methane, hydrogen sulfide, carbon dioxide, and nitrogen. This stage in decomposition is termed putrefaction.
The accumulation of gases that are being generated within the body’s dead tissue and cells but not able to initially completely break through the surface of the skin or the various pockets of contained spaces within the body leads to some build-up of pressure.
This pressure due to the expansion of the gases leads to the swelling or bloating of the cadaver to accommodate the expanding volume of gas.
This gas build-up also causes fluids remaining in the cadaver to become foamy and much of it being forced out of any available orifices. It sometimes eventually leads to a rupture in part of the body which releases some of the accumulated gas.
Hemoglobin is an iron-containing metalloprotein (a generic term for a protein that contains a metal ion cofactor {a substance other than the substrate (the substance on which an enzyme acts) whose presence is essential for the activity of an enzyme}) contained in red blood cells that carries oxygen from the lungs to the rest of the body.
It is responsible for the red pigment in the blood and is changed or transformed into sulfhemoglobin (a stable green pigmented molecule that is generated from the oxidation of the iron in hemoglobin to a ferric {containing iron II}state by chemicals and substances that contain sulfur) and other dark discolored pigments by intestinal anaerobic bacteria.
The sulfhemoglobin is further transported around the body by the gases that are being generated through the circulatory and lymphatic systems that aid circulation during the vertebrate’s lifetime.
For carcasses that are exposed, there is also often heavy scavenging by animal scavengers like vultures, hyenas, coyotes, crocodiles, carrion crows, etc depending on the region and infestation by multiple insects like ants, parasitoid wasps, flies, beetles, pillbugs, crickets, and numerous others, some arachnids, and other organisms.
Some insects like blowflies and others lay eggs on the surface of the cadaver or in any available orifices where they soon hatch and their larvae (maggots) begin to rapidly consume the remains.
They move throughout the body spreading bacteria and secreting their own digestive enzymes and ripping into weakened tissues with their mouth hooks which can cause breaking or rupturing of the skin releasing accumulated gases and fluids then collapsing the body structures and accelerating the decay process.
Ruptures and breaks that occur in the bloated cadavers that are being acted upon allow the reintroduction of oxygen and introduction of different types of insects and other organisms into the carcass that enables the renewed action of aerobic microorganisms and maggots.
Active Decay
The active decay stage is the period that has the greatest reduction in the overall body mass of the cadaver. The change in the size and surface area of the body is clearly visible at this stage because the process of decay is at its peak.
These processes and their combined effects especially putrefaction has rendered the remaining tissues and soft structures of the carcass devoid of the bonds, cohesiveness, and elasticity that previously held the constituents of the body in an integral whole.
Consequently, the constituents of the body are loosely placed with many parts virtually falling off or falling apart and liquefying (solid and semi-solids becoming fluids) thereby making the digesting, absorbing, evaporation, spilling, and withering away to the atmosphere easier and faster.
The bodily fluids that seep out percolate to the surrounding surfaces and are visible around the cadaver creating a cadaver decomposing island (CDI).
The active decay stage can be gauged to be ending when the presence of maggots reduces or ends due to either their maturing and leaving the body or migrating away from it before maturity.
Advanced Decay
The advanced stage of decay is characterized by an extremely diminished body mass availability. Most of the carcass has been consumed or wasted away by a wide range of organisms and conditions including seepage and evaporation of bodily fluids.
These conditions and organisms have not only consumed or carried away parts of the carcass but have aided its reduction in size by breaking it down into smaller masses that are easier to liquefy, disintegrate, disperse, or digest.
Due to these factors, there is also less fluid available for the high level of microbial activity obtainable in the previous stage. Many of the other organisms like many of the insects have also left the carcass or greatly reduced their activity.
Only a few desperate scavengers will visit at this point particularly those specialized enough to deal with drying or difficult-to-reach parts like hyenas breaking bones to reach the marrow or vultures using their sharp beak and talons to strip shreds of drying flesh, cartilage, or skin from bones.
Any vegetation around the (CDI) cadaver decomposing island will typically have signs of soil and grass discoloration and withered foliage from the dead or sickly plants affected by the chemically altered bodily fluids.
The soil will generally have a change in its pH and an increase in elements like nitrogen and carbon and nutrients like magnesium, phosphorus, potassium, and calcium.
Dry Remains
The dry remains stage of decomposition is the last stage of decay and is characterized by the absence of any remaining soft tissue on the cadaver. There is typically just dry skin, remnants of cartilage, possibly some dried-out tendon and ligaments, some hair, and bones.
When the cadaver is at this stage and all the soft tissue has been completely removed or degraded, it is referred to as totally skeletonized.
However, when there are still some parts of the carcass that have some drying or dry soft tissue like tendons, cartilage, and ligaments or a notable amount of skin, then it is said to be partially skeletonized.
At this stage organisms like tineid moths (also called fungus moths from the order Lepidoptera that includes several economically important species of clothes moths) and micro bacteria feed on remaining parts like the hair.
These organisms are in turn sought and fed on by mites (arachnids that are members of the subclass Ascari). They can be found as long as there is still some traces of hair.
Abiotic Decomposition

Abiotic decomposition is the degradation or breakdown of inanimate objects (inorganic objects that cannot grow, reproduce, or self-locomote due to the absence of life) and other substances that constitute non-living components of an ecosystem by physical degradation processes or chemical reactions such as hydrolysis.
Processes of Abiotic Decomposition
They may either be natural occurrences such as weathering and fragmentation of rocks in the physical process and chemical decomposition in the chemical reaction that occurs naturally or artificially executed chemical reactions.
Processes like rusting of metals and the decay of synthetic materials like plastic are also forms of abiotic decomposition.
Physical Process
The physical processes of abiotic decomposition/degradation (stage by stage breaking down of a compound or object) are the weathering (gradual weakening and reduction in structural mass and integrity typically due to mechanical processes like the impact of climate), systematic breakdown, and fragmentation (systematic break down of overall size of an object or cohesive substance into small bits) of the physical attributes of the abiotic or non-living components that have any form of structural integrity (cohesive strength of a unit/structure) in an ecosystem.
Many instances of this physical degradation are due in addition to mechanical exertions, directly or indirectly to preceding chemical reactions that result in physical degradation.
Physical changes may occur to the structure, physical composition, and form of a chemical substance or object without affecting or altering its chemical composition or configuration.
The weathering, fragmentation, and sedimentation of large rocks create smaller ones that may still undergo weathering over time and keep being broken up gradually becoming smaller until the smaller sediments become part of the soil.
Decomposition in an Ecosystem
Decomposition is undoubtedly one of the most important processes that are naturally carried out as a basic foundation to support the food chain and therefore very crucial to the existence of the ecosystem.
The series of processes through which the carbon and nutrients in dead organic matter are broken down is known as decomposition. Without decomposition, the energy and nourishment would not be able to be absorbed by other organisms and dead plants and animals would not be broken down.
This could potentially cause environmental contamination, pollution, the spread of disease, and litter the ecosystem. Decomposition releases nutrients that can be re-used for plant and microbial production. It also returns carbon dioxide to the atmosphere and water where it can be used in photosynthesis.
In the absence of decomposition, dead organic matter will continue to accumulate in the ecosystem the nutrients and atmospheric carbon dioxide will be depleted. Roughly 90% of terrestrial net primary production goes from plant to decomposer.
Decomposition Process
The decomposition process recycles organic nutrients, minerals, and energy. This process is crucial for the continued availability of these resources to support the biotic and abiotic systems. The process can be divided into five main phases based on their individual natural actions which are outlined as follows:
- Fragmentation
- Leaching
- Catabolism
- Humification
- Mineralization
Fragmentation
Fragmentation is the first stage of decomposition when masses of detritus or larger cohesive units of organic material are divided into smaller pieces by the detritivores making them easier for further decomposition and dissolution.
The process of fragmentation (breaking up into small pieces) can also be described as reducing whole sections of organic material into smaller segments thus exposing new surfaces that can be acted on by microbes.
A lot of organic material that has been shed, died, or otherwise made available for decomposition may contain a protective layer or layers of material that can hinder microbial action.
Some of these materials could be coatings of cuticle, chitin, bark, skin, cell walls, or an exoskeleton. The fragmentation process acts on and breaks through these protective layers and exposing the nutritious inner material to accelerated microbial decomposition.
Ruminant animals foraging for food also help to fragment detritus (fragmented particles of dead organic matter) including passing through their digestive system when they ingest it. Freeze-thaw and wetting-drying cycles also contribute to fragmentation in an ecosystem.
Leaching in Decomposition
As water moves through dead organic material, the water-soluble components become dissolved and carried along with it. This is termed leaching.
These are then either absorbed by the organisms in the soil, react with the mineral components of the soil, or are transported and redistributed within or beyond the confines of that particular biome or ecosystem.
High concentrations of water-soluble (easily dissolved in water) components including sugars, amino acids, and mineral nutrients are contained in newly shed leaves and recently deceased animals. Leaching is more important in wetter environments and ecosystems than dry ones due to its requiring fluid.
Catabolism
Catabolism is the complex metabolic process of certain fungal and bacterial enzymes synthesizing the fragmented and dissolved detritus materials and converting them to simpler inorganic compounds. The products can either be oxidized to produce energy or utilized in other anabolic (sequence of metabolic pathways that construct molecules from smaller units of matter) reactions.
The process of catabolism decomposes large molecules like polysaccharides, proteins, lipids, and nucleic acids into smaller basic units like amino acids, monosaccharides, nucleotides, and fatty acids.
The Chemical Alteration of Dead Organic Matter

The chemical alteration of dead organic matter is primarily achieved through bacterial and fungal action. Fungal hyphae (a cluster of long branching filamentous structures of a fungus, oomycete, or actinobacterium) produce enzymes that can penetrate the tough outer structures encasing dead plants or animals.
They also produce a type of enzyme that breaks down lignin. This enables them to get access to the inner parts of the cell and the nitrogen contained in the lignin. Fungi have the capability to transfer carbon and nitrogen through their hyphal network (structural collection of fungal hyphae) and are not completely dependent on local resources like bacteria.
The chemical alteration of dead organic matter also depends largely on the rate of decomposition obtainable in the particular area in which it is occurring.
The rate of microbial respiration (the exchange of gases between soil-dwelling microbes and the environment) is determined mainly by temperature, therefore, faster microbial decomposition occurs with higher temperatures.
Humification
Humification is the complex natural process of converting organic matter into amorphous humic substances (humus, humate, humic acid, humin, and fulvic acid) by geo-microbiological mechanisms and the actions of a combination of organisms such as saprophytic fungi, bacteria, microbes, earthworms, nematodes, protozoa, and arthropods.
The humification process shares a geochemical link with the mineralization process that occurs after the decomposition of detritus during the nitrogen and nutrient cycles.
A significant fraction of the nutrients undergoing mineralization while being recycled back into the system do not become mineralized but become transformed by humification into a linked series of stable organic polymers that constitute humus.
Mineralization
Mineralization is the final stage in the process of decomposition and is the oxidation reaction that transforms complex chemical compounds through decomposition into simple organic matter.
The process makes nutrients that were hitherto either too complex or inaccessible for reabsorption simplified and available. Large volumes of phosphorus, sulfur, and nitrogen are produced and mineralization is the opposite of the process of immobilization (the conversion of inorganic compounds to organic compounds by microorganisms and plants).
Mineralization generally only occurs as far as the concentration of an element exceeds the requirements of its decomposer for biosynthesis (a complex organic process hastened by enzymes where substrates are converted into more complex products in living organisms) or storage.
Factors That Affect The Rate of Decomposition
Ecosystems generally have varying rates of decomposition which can be determined by a few factors namely:
- Litter Quality
- Temperature
- Aeration
- Soil pH
- Inorganic Chemicals
- Moisture
Litter Quality
The structural and chemical properties of litter (layer of dead plant material on the soil’s surface) generally determine the rate of decomposition. When the process involves the degradation of tough materials like the litter of bryophytes (non-vascular seedless plants including mosses, hornworts, and liverworts) due to the presence of lignin (a class of complex organic tissue-building structural polymers)-like complex chemicals in its thalli (microscopic branching filaments).
Temperature
Temperature is a pivotal factor in the general activities of microorganisms including decomposition. Extreme temperatures can significantly slow down the process of decomposition whereas the ideal temperature conditions catalyze the process.
Aeration
The availability of oxygen in the soil to aid microorganisms in their growth and aerobic metabolism process is another factor that determines the rate of decomposition. In the absence of oxygen, only anaerobic organisms are able to grow and decompose materials by digestion which takes a much longer time.
Soil pH
The specific measure of the hydrogen ion concentration or the specific acidity or alkalinity of the soil (the presence of cations and anions) is referred to as the soil pH and is a determining factor in the growth of microbes.
Inorganic Chemicals
The presence of previously decomposed inorganic elements in the soil also affects the rate of decomposition due to the microorganisms needing them for growth.
After the process of decomposition elements like potassium, calcium, magnesium, and sodium are released into the soil where they’re used by microbes.
Moisture
Water is generally instrumental for most biological processes and in decomposition the presence of water greatly accelerates the process. The absence of moisture retards microbial activity.
Decomposing Body
As mentioned in the preceding text, the decomposition of a vertebrate is the systematic breaking down and decay of the physical remains of a recently deceased animal.
This natural phenomenon is aided by chemical and physical reactions through the action of the environment, microbes, and other organisms in a process that recycles minerals and organic matter back into the ecosystem.
This process also applies to a human or animal body with the duration and intensity largely dependent on its body mass and weight. The decaying process consists of five main stages from onset to finish and is given as; autolysis, bloat, active decay, advanced decay, and dry remains.
There may be further decomposition after the main stages depending on the given conditions but that is not generally included in the analysis of the basic decay of a body.
How Long Does It Take a Body To Decompose?
The whole process of decomposition starts from autolysis at the fresh stage immediately after the heart stops beating through the liquefaction of tissues during the active decay, to the beginning of skeletonization at the dry remains stage.
The whole process may take between twenty to thirty days depending on a few factors but especially on the atmospheric conditions and the location of the cadaver.
Factors Affecting the Decomposition Rate of a Body
The rate of decay of any animal or organism including vertebrates like humans who have larger and denser bodies depends on certain factors that may either accelerate or retard the process of wasting away by either enhancing conditions that promote bacterial, parasitic, and microbial activity or hindering/limiting them.
The main factors that determine the rate of decay are as follows:
- Exposure to the elements– The most significant determining factor that possibly has the most impact on how rapidly and thoroughly a body will decay is whether it is exposed to the effects of climate. Bodies that are exposed to nature and not encased in material or a medium nor buried will have a much faster rate of degradation than any that are either put below the ground or those that are located in a building, structure, or vessel. This is due to the effects of sunlight, rain, wind, air, and easy access by animals and organisms especially flies.
- Temperature– The ambient temperature of the location of a body is also crucial to the rate of decay as it affects the ability of animals and microbes to carry out their digestive and degrading activities. Generally the lower the temperature, the lower the rate of decay.
- Availability of oxygen-The presence of oxygen is important especially at the initial stage for aerobic microbial action. It is also needed by many parasitic organisms like insects and others that require it to operate.
- Cause of death– Cause of death can also have a major effect on the rate of decomposition because certain diseases cause serious internal damage that can accelerate the process. Injury and trauma to the body antemortem (before death) can already be infected and speed up the decay process postmortem (after death).
- Embalming or preservative treatment– Corpses that have been deliberately or accidentally exposed to or treated with some form of preservative herbs, substances, or compounds will have an impeded degeneration process.
- Trauma or laceration postmortem– Injury or trauma after death from dropping the corpse, scrapes from moving it, or action of wild animals can also provide an easy entry for saprophytic (organisms that feed on dead and decaying matter) organisms and hasten the wasting process.
- Burial-Whether or not a cadaver is buried, the type of soil it is buried in, and how deep it is buried all directly affect how fast or slow the body will decay.
- Scavenger access-The action of scavengers greatly increases the rate at which the cadaver will be broken down. Consequently, the corpse will be broken down faster if scavengers have access to it.
- Humidity/moisture-Moisture generally promotes the presence and action of bacteria. It acts as a medium of infestation and transportation around the body thereby increasing the rate of decay.
- Rainfall– Rainfall increases the general moisture around the corpse increasing the conditions suitable for increased microbial action. Acid rain (or acid deposition is any kind of precipitation that contains any form of acidic substances such as sulfuric or nitric acid that descend from the atmosphere in either dry or wet forms including; rain, fog, snow, dust, or hail) and other acidic precipitation also speedens up the decay process of a cadaver.
- Body mass– The body mass of a corpse can also affect the rate of decay as a denser body mass index will require more and longer degrading actions to break down.
- Clothing or cover– The type of fabric or other covering the cadaver is wearing or covered with can also slow the access to the tissues that potential scavengers or parasites have.
- Resting surface– The type of surface a body is lying on can speeden up or slow down the wasting process. Cadavers that are making contact with soil will decompose much faster with microbes and other organisms moving freely between the soil and the body and rapid drainage of fluids than one placed or lying on concrete or synthetic materials where ground-dwelling microorganisms won’t have unhindered access.
The table below shows the average timeline of the decomposition of a body
Time Postmortem |
Effect on Corpse |
5 to 30 minutes |
Paling of the skin pallor mortis (visible) and the disintegration of cell membranes triggering autolysis from lack of circulation (invisible) |
24 to 72 hours |
Internal organs decompose (visible from abdominal discoloration) |
3 to 5 days |
The body begins to swell and bloat with drooling of foamy and putrid bodily fluids and from orifices and any wounds due to pressure build-up pushing them out (visible) |
8 to 10 days |
A transition of discoloration from greenish to reddish with dark areas. Abdominal accumulation of gas. (visible) |
14 to 21 days |
The withering and falling off of dead cells like hair and nails and often teeth as well (visible) |
30 to 35 days |
The body starts to liquefy and lose form (visible) |
35 days + |
Hardly any visible remains other than bones and remnants of cartilage, hair, and some skin. (visible) |
Types of Chemical Reactions in Decomposition
Chemical Reaction
A chemical reaction is an interactive process in which reactant substances undergo changes that convert them to one or more different product substances. The changes in the physical attributes and general make-up of these reactants are achieved by the rearrangement of their constituent atoms.
A chemical reaction can also be defined as an interactive process on the molecular level where one or more chemical elements, given substances, or compound reactants have their constituent atoms rearranged or converted to produce different derivatives of the initial compounds.
Chemical changes are sometimes reflected in corresponding physical changes while others differ from physical changes that only affect the physical state of the compound or substance but not the chemical constituents.
A lot of chemical reactions play an important role in the process of decomposition and chemical decomposition is a type of chemical reaction.
There are several types of chemical reactions that play important roles in the numerous simple and complex functions of the environment and the entire universe at large.
The main types of chemical reactions that are associated with decomposition are outlined below:
- Decomposition reaction
- Double decomposition reaction
- Oxidation reaction
- Combustion
Decomposition Reaction
Decomposition reactions can be defined as the process whereby a complex compound is divided and reduced to two or more simpler components on a molecular level. This is usually triggered by the input of an external influence like energy in the form of light, heat, or electricity.
Generally, decomposition reactions can be either exothermic (involving the release of energy usually in the form of heat or light).
Or they can be endothermic (involving the transfer of externally derived heat that is absorbed by the reactants resulting in the cooling of their immediate surroundings). However, endothermic reactions are more prevalent in occurrence in regard to decomposition reactions.
Decomposition Reaction Formula
AB → A + B
With AB representing the parent compound reactant and A + B representing the broken down molecules.
Examples of decomposition reactions are;
- Production of oxygen in a laboratory from the decomposition of potassium chlorate using heat energy.
- The decomposition of carbonic acid in carbonated soda drinks.
- The electrolysis (a process of decomposing ionic compounds into their elements by passing a direct electric current through the compound in a fluid form. The cations are reduced at the cathode and the anions oxidized at the anode) of water that yields oxygen and hydrogen.
Types of Decomposition Reactions
- Thermal decomposition reaction
- Electrolytic decomposition reaction
- Photo/photolytic/photochemical decomposition
Thermal Decomposition Reaction
Thermal decomposition reactions also called thermolysis (the break down of molecules by heat action) are reactions that are triggered by thermal or heat energy.
The reactant requires exposure to, or injection of heat for its bonds to be broken into two or more simple derivative compounds. The decomposition temperature is the temperature required to chemically decompose a given compound or substance.
These types of reactions are generally endothermic because they absorb ambient heat from around the reaction site thereby having a cooling effect on the surrounding. An example of a thermal decomposition reaction is when calcium carbonate that is exposed to heat decomposes to produce calcium oxide and carbon dioxide.
Electrolytic Decomposition Reaction
An electrolytic decomposition reaction is a type of decomposition reaction that utilizes electrical energy to trigger the reaction. This type of decomposition reaction is achieved by passing an electric current through an aqueous solution of the compound to be broken down. The equation for an electrolytic decomposition reaction is given as:
AB electricity→ A + B
Photolytic Decomposition Reaction
Photolytic, photo, or photochemical decomposition or simply photolysis is a type of decomposition reaction in which the parent reactant is divided into its individual constituent products by absorbing energy made available by photons (very small particles that are constituted by waves of electromagnetic radiation) or light energy. The light triggers or excites the electron in the parent reactant.
Decomposition Reaction Examples
The following are examples of the various types of decomposition reactions.
Example of a Thermal Decomposition Reaction
Calcium is heated to decompose into calcium oxide and carbon dioxide. This process is used in the production of calcium oxide which is commonly called quick lime. This reaction is illustrated in the equation;
CaCO3 → CaO + CO2
The breakdown of copper carbonate to produce copper oxide and carbon dioxide. This thermal decomposition reaction is represented by the following equation;
CuCO3 → CuO + CO2
Examples of Electrolytic Decomposition Reactions
An example of electrolytic decomposition is the electrolysis of water illustrated by the equation;
2H2O → 2H2 + O2
The decay reaction of sodium chloride occurs when electricity is passed through an aqueous solution of sodium chloride (NaCl) forming chlorine and sodium. Electrolysis of sodium chloride is shown in the equation;
NaCl → Na + Cl
Example of a Photolytic Decomposition Reaction
An example of a photochemical decomposition reaction is the breakdown of silver chloride from exposure to light energy to produce silver and chlorine represented by the equation;
2AgCl → 2Ag + Cl2
Double Decomposition Reaction
A double decomposition reaction is a form of a double displacement, double replacement, or metathesis reaction where two constituent reactant compounds (cations and anions) interchange or inter-switch positive and negative ions forming new compounds in the process.
This is just as obtainable with a double displacement reaction except that in this reaction, one or more of the reactants does not dissolve in the given solvent (a liquid in which a solute is formed to constitute a solution).
The term double decomposition reaction is an older term and has largely been replaced by double replacement reaction but is still used to refer specifically to metathesis reactions that involve one or more reactants that are not solutes and therefore don’t dissolve in the solvent medium.
Double Decomposition Reaction Formula
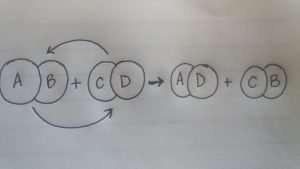
The double decomposition reaction formula can also be represented by the simple formula below.
AB + CD → CB + AD
Hydrolysis
Hydrolysis is basically a double decomposition reaction that can be described as a chemical process of decomposition that involves the splitting of a covalent bond (inter-atomic connection due to atoms sharing an electron pair) with the insertion of a hydrogen cation (positively charged ion) and hydroxide anion (negatively charged ion) of water.
The term ‘hydrolysis’ is often broadly used for chemical reactions in which water is the nucleophile (a chemical entity that establishes bonds with electrophiles when it transfers an electron pair). Such reactions include elimination, solvation, and substitution reactions.
Biological hydrolysis involves the separation of biomolecules (microscopic units of biological compounds produced by cells and living organisms including the four major ones; carbohydrates, lipids, nucleic acids, and proteins) in a reaction where a water molecule is utilized for the division or decomposition of a larger molecule into derivative components.
Hydrolysis reactions are typically regarded as being the direct reverse or opposite of condensation reactions due to the inverse similarities in their fundamental processes. The insertion of water for decomposition by the former and the ejection of water for composition by the latter.
Polysaccharides and Saccharification
Polysaccharides are also referred to as polycarbohydrates and are the most abundant carbohydrate found in food. They are constituted of monosaccharides (simple sugars that constitute the building blocks of carbohydrates) monomers (atoms or small molecules bonded together forming more complex structures like polymers) or units.
They form lengthy polymeric (substance with macromolecules consisting of several identical subunits) chains that are bonded by glycosidic (a covalent bond between a carbohydrate and another entity) links.
The decomposition of polysaccharides into soluble sugars through the process of hydrolysis is considered a form of saccharification (a chemical change in which a monosaccharide molecule remains unaltered after decomposition from another saccharide to which it was bonded).
Oxidation-Reduction Reaction/Redox Reaction
An oxidation-reduction or redox reaction is a chemical reaction in which there is a transfer of electrons between two chemical species and in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron.
The actual oxidation state (degree of oxidation or oxidation number refers to the electrons lost by an atom in a given chemical compound) of an element directly corresponds to the number of electrons that an atom may lose, gain, or seem to expend or use up when combining with other atoms in given compounds.
Redox reactions are comprised of two stages of interaction that occur simultaneously. The reduced half gains additional electrons and reduces or decreases its oxidation number, while the oxidized half loses electrons and gains or increases its oxidation number.
Redox reactions are important in several processes like photosynthesis, respiration, combustion, and decomposition processes like corrosion and rust.
Oxidation-reduction/Redox reaction formula
Reduction
Oxidant + e– → Product
(Gains electrons) (oxidation number decreases)
Oxidation
Reductant → Product + e–
(Loses electrons) (oxidation number increases)
Combustion
Combustion can simply be defined as the process of a material or substance burning. It is an exothermic oxidation-reduction chemical reaction at high temperatures between a reductant (the fuel) and an oxidant (typically atmospheric oxygen) that generates oxidized and sometimes gaseous fumes and other by-products in a thick discharge generally called smoke or fumes.
Combustion does not necessarily always involve conventional fire as some combustion reactions occur with an absence of flames which are generally only visible when the reductant or fuel vaporizes and with suitable conditions in place can occur in mediums like water and vacuums like outer space.
It however always causes or greatly accelerates the process of decomposition and typically alters the constituent properties of the combusting substance often consuming some of the material while breaking them down to their most basic form which is carbon and ash.
Incineration
Incineration is the high-temperature combustion (rapid oxidation) of a material such as waste or other materials for the purpose of decomposing them to a required degree.
Incinerator
An incinerator is a furnace or a containment vessel or apparatus for the controlled combustion or burning of specific materials at high temperatures to decompose them for various purposes.
Combustion Reaction
A combustion reaction is a chemical reaction in which a substance or material reacts with oxygen and releases energy in the form of light and heat.
Combustion Reaction Example
In this instance, the equation below illustrates how the combustion of hydrogen gas produces water vapor.
2H2 (g) + O2 (g) → 2H2O (g)
Pyrolysis
Pyrolysis or devolatilization is the heating of certain organic materials such as biomass at very high temperatures anaerobically or in the absence of oxygen which results in a change in its chemical composition with no visible conventional combustion occurring.
Instead, there is a thermal decomposition (or thermolysis a chemical decomposition caused by heat) of the constituent chemical compounds like hemicellulose, cellulose, and lignin into combustible gases and charcoal.
Uses of Decomposition reaction
- Thermit welding (the process of exposing a set of different individual high-energy metals [also called thermite] to intense heat to produce a molten metal that is used as a welded joint for other metals).
- It is used for the relief from acid indigestion.
- The manufacture of calcium oxide (cement).
- It is used in metallurgy (materials and engineering science that study metallic elements’ physical and chemical behavior, their inter-metallic compounds, and their alloys).
Combination Reaction/Synthesis Reaction
A combination or synthesis (interactive combination of two or more elements to form a cohesive unit) reaction is technically the opposite of a decomposition reaction and involves the formation of a complex chemical compound (the product) from the reaction of two or more previously independent and simpler reactant compounds.
A decomposition reaction where a complex chemical compound is divided into simpler individual compounds is typically a reversable reaction. If the reaction is reversed, the individual simple compounds will synthesize or combine to form a single complex compound.
The reaction that initiates and causes the reverse reaction to that of a decomposition reaction is a combination reaction. This is the relationship between the two types of chemical reactions.
Combination Reaction Formula
X + Y → XY
X and Y represent the parent simpler reactants where XY represents the single complex product of the reaction.
Combination reactions are usually generally exothermic (involving the release of energy usually in the form of light or heat) due to the heat that is released from the action of bonding by the reactants. Examples of combination reactions are:
- Iron combines with sulfur to produce iron (II) sulfide.
- Barium metal and fluorine gas combine in an exothermic reaction to yield salt barium fluoride.
- Magnesium oxide combines with carbon dioxide to produce magnesium carbonate.