Table of Contents
What is a bacteriophage?
A bacteriophage is a bacterial virus that infects bacteria and archaea. They are also called phages or bacterial viruses in microbiology. The term bacteriophage means bacteria eater and this term was coined by Félix d’Hérelle. Bacteriophages were discovered independently in 1915 by Frederick W. Twort in Great Britain and in 1917 by Félix d’Hérelle in France.
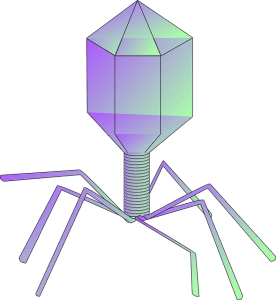
These viruses infect and replicate in archaea and bacteria, destroying their cells. They are composed of a nucleic acid molecule, either DNA or RNA that is surrounded by a protein structure. The genome of these bacterial viruses may encode genes ranging from four to hundreds of genes. They replicate in the bacteria after the injection of their genome into the cytoplasm of the host. When a bacteriophage gets a host bacterium, they attach to it and infect the host cells.
After infection, they take over the cellular system of the bacteria. Hence, preventing it from generating bacterial elements. Rather, the cellular system produces viral components. Then, new bacteriophages assemble and burst out of the bacterium. This process is called lysis. Occasionally, these bacteriophages carry out a process during infection called transduction. In transduction, they remove a part of the host cells’ DNA and sends it into the genome of new host cells.
What is a phage?
A phage is a short form of bacteriophage. This term is informally used. Phages are viruses that infect bacteria. Bacteriophages or phages are interestingly the most diverse and common entities on earth. They are ubiquitous viruses that are seen wherever bacteria exist.
There is an estimation that there are more phages than any other organism on earth. Bacterial viruses are even more than bacteria in the biosphere. Viruses happen to be the most abundant biological entities in the oceans, globally. After prokaryotes, they are the second-largest element of biomass. Approximately, 70% of aquatic bacteria may be infected by bacteriophages.
Since the late 20th century, bacterial viruses have been used as an alternative to antibiotics in certain places like France, the former Soviet Union, and Central Europe. These viruses are studied and seen as a possible therapy against multi-drug resistant strains of several bacteria. There are so many varieties of phages out there and each infects only one type or a few types of archaea or bacteria.
Classification
Bacteriophages are classified in several virus families such as:
- Myoviridae
- Siphoviridae
- Podoviridae
- Lipothrixviridae
- Rudiviridae
- Cystoviridae
- Fiersviridae
- Microviridae
- Inoviridae
- Corticoviridae
- Finnlakeviridae
- Tectiviridae
What do bacteriophages infect?
Bacteriophages infect bacteria and archaea. They use their tail to stab and pierce through the membrane of the bacterium in order to infect it. These tails are made up of a contractile sheath surrounding a tube. Once the phage attaches to the surface of the bacteria, the sheath contracts and moves the tube through it. At the end of the tail, there is a million-atom baseplate structure that controls this.
Bacteriophages are diverse in the biosphere and are seen anywhere bacteria are. They are found in water, soil, algal bloom, hot springs, animals, etc. These viruses however have a severe impact on the diversity of the bacterial populations. Moreso, there are countless bacteriophages in the human body, though they don’t infect humans. They exist in humans basically because of the extensive microbiome in humans.
The population of phages in humans is called the human phageome. There is the healthy gut phageome (HGP) and the diseased human phageome (DHP). However, the role of phages in human health is clearly not understood. Although studies have shown that common phages are seen on an average in 62% of healthy humans. Whereas, their occurrence was reduced by 54% and 42% in patients with Crohn’s disease and Ulcerative colitis. Due to this study, it is indicated that bacteriophage may be crucial in the human gut microbiome to regulate the population of both harmless and pathogenic bacteria.
Bacteriophage structure
Like every other virus, bacteriophages are simple entities that consist of genetic nucleic acid surrounded by a protein capsid. This nucleic acid may be double-stranded or single-stranded and may be either RNA or DNA. However, bacteriophages exist in either of these 3 basic structural forms:
- A filamentous form
- Icosahedral (20-sided) head with a tail
- An Icosahedral head without a tail
The filamentous bacteriophage belongs to the virus family Inoviridae. They are worm-like and about 1000-2000nm long with an approximate diameter of 6nm. Their coat is made up of 5 types of viral protein. These proteins are located in the inner membrane of the host bacteria during phage assembly.
A virus’s shape is closely relative to its genome. Hence, a large genome indicates a large capsid (head) and a more complex organization. The bacteriophages with tails are the most studied and largest group of phages belonging to the order Caudovirales. These tailed phages are grouped based on their type of tail into Siphoviridae, Podoviridae, and Myoviridae.
The tailed phages with long non-contractile tails belong to the family Siphoviridae. Whereas, those that have a short non-contractile tail belong to the family Podoviridae. The tailed phages with complex contractile tails belong to the family Myoviridae. There are 3 major components in tailed bacteriophages:
- A capsid head where the genome is packed
- The tail that serves as a pipe to transfer its genome into the host cell during infection
- An adsorption apparatus at the end of the tail for recognizing the host cell and penetrating its wall.
Bacteriophage Diagram
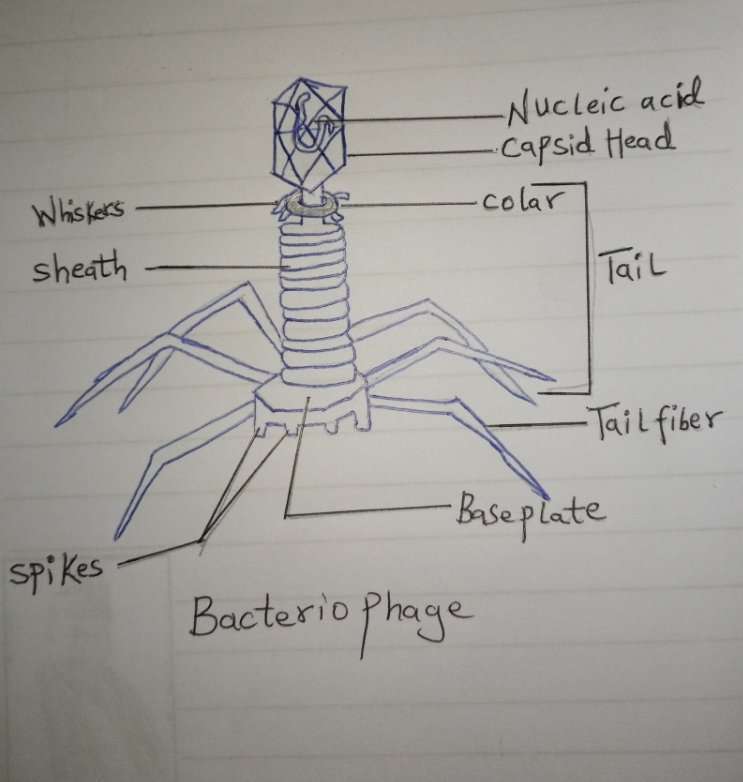
Underneath the capsid is the collar. All bacteriophages have a head structure (capsid). Inside the head of the phage, lies the nucleic acid (DNA or RNA), and the head (Capsid) serves as a protective covering. The capsid head of the bacteriophage usually has icosahedral of fivefold symmetries. These symmetries are broken down by the head-to-tail interface (HTI) at one of the fivefold axes. The main element of the head-to-tail interface is a dodecameric portal protein (PP) in the capsid. A dodecamer is a protein complex consisting of 12 protein subunits. This portal protein (PP) serves as the DNA-packaging motor. Also, the head-to-tail interface (HTI) includes oligomeric rings of head completion proteins. These oligomeric rings carry out 2 major roles:
- Makes an additional interface to molecules of ATP that supply energy for DNA packaging
- Connects the portal protein and the tail
Some HTIs also act as valves that close the exit channel. Hence, preventing leakage of the genome from the capsid. Though it opens when the bacteriophage is attached to a host cell. Additionally, symmetries other than dodecameric have been seen for almost all portal proteins (PPs) in vitro. This happens when the PPs are assembled under natural conditions without any other bacteriophage protein components.
Aside from the capsid, the phage tail is a very essential structural component of the phage. It is very crucial during infection. These tails are made up of a contractile sheath surrounding a tube. Once the phage attaches to the surface of the bacteria, the sheath contracts and moves the tube through it. At the end of the tail, there is a million-atom base plate structure that controls this.
Procapsids
The procapsid is the precursor of the capsid (head) of the bacteriophage during the assembly process. However, there are scaffolding proteins (SPs) that drive the assembly process. They accompany major capsid protein (MCP) subunits to build an icosahedral procapsid. This icosahedral procapsid is filled later with double-stranded DNA (dsDNA). Immediately the procapsid is assembled, the scaffolding domain is slit off and will be removed from the capsid to give space for the genome.
Capsids
All bacteriophages have a head structure (capsid). Although their capsid varies in size and shape. Some phages have icosahedral capsids while others are filamentous. However, a typical phage has a hollow head that contains the phage DNA or RNA. The capsid is made up of multiple copies of one or more proteins.
Inside the head of the phage, lies the nucleic acid, and the head (capsid) serves as a protective covering. However, the nucleic acid of bacteriophages contains modified bases that protect its nucleic acid from nucleases. These nucleases break down the nucleic acid of the host during infection.
The size of the nucleic acid in a phage varies based on the phage. The simplest bacteriophage has nucleic acid enough to code 3-5 average size gene products. Whereas, the complex bacteriophage has nucleic acid enough to code more than 100 gene products.
Likewise, the different kinds of protein and the amount in a phage varies based on the bacteriophage. The simplest bacteriophage has many copies of only about 1 or 2 different proteins. Whereas, the complex bacteriophage may have many copies of different kinds of protein. These proteins have a crucial role to play during infection. They protect nucleic acid from nucleases.
Connectors
In bacteriophages and herpesviruses, one of the fivefold vertices of the capsid is replaced by a head-to-tail interface (HTI). This HTI is a multi-protein complex (connector).
In all bacterial viruses, the head-to-tail interface (HTI) gives a platform for docking of preassembled tails in Myoviridae or Siphoviridae. Also in Podoviridae, it initiates the assembly of a short tail.
The HTI however, is made up of a portal protein complex (PP) and head completion proteins. These proteins act as a valve for closing the channel and keeping the genome of the bacteriophage inside the capsid at high pressure. They only open immediately the phage gets tightly attached to a host cell to permit genome release from the capsid under natural conditions.
Tails
The tail organization in phages varies based on their type.
- Siphoviridae possess long flexible tails
- Podoviridae have very short tails that mostly contains the adhesive device
- Myoviridae possess rigid long contractile tails that are made up of a number of different proteins that form the inner rigid tube and the outer contractile sheath
However, Siphoviridae and Myoviridae have an independent pathway for the assembly of their tails. The tails of these phages are attached to the capsid after it has been packed with the genome. Whereas, in Podoviridae, after DNA packaging, the tails are assembled on the capsids as the last step of self-assembly.
The family Siphoviridae has long tails that are made up of tail proteins (TPs). These proteins form circular oligomeric rings with threefold or sixfold rotational symmetry. The rings are coupled around a tape measure protein (TMP) that defines the length of the tail. Thus, these rings are stacked on top of each other with helical symmetry.
Then when the phage tail reaches the length determined by the tape measure protein (TMP), a tail terminator protein (TrP) caps the tail. This tail terminator protein (TrP) acts as an interface with the capsid.
Hence, when the bacterial virus interacts with the host receptor, the head-to-tail interface (HTI) opens. Then, the tape measure protein (TMP) is pushed out by the DNA due to the inner pressure of the capsid. Most long tails of the bacteriophage have a smooth outer surface while some have attachments that stick outwards from the tail surface.
Adsorption apparatus
Located at the distal end of the tail is the adsorption apparatus. This special adhesive system recognizes the envelope chemistry or receptor of the host cell. It ensures the delivery of the phage genome to the cytoplasm of the cell. The tail in Siphoviridae and Myoviridae is made up of series of stacked rings with the adsorption apparatus located at the end of the tail. Whereas, the adsorption apparatus is immediately bound to the Head-to-tail interface (HTI) in Podoviridae. However, in many bacterial viruses, the adsorption apparatus is surrounded by fibers that guarantee a tight link to the host cell.
The majority of phages in the family Siphoviridae have an oligomeric ring that is formed by distal tail proteins (DTPs). This oligomeric ring is attached to the last ring of the tail tube. The distal tail proteins (DTP) ring, however, acts as an apparatus to recognize and connect to receptor-binding proteins. Oligomeric rings carry out 2 major roles: make an additional interface to molecules of ATP that supply energy for DNA packaging and connects the portal protein with the tail.
This interaction sometimes is aided by tail fibers as seen in some phages. Furthermore, several bacteriophages that attack gram-negative bacteria possess lysozyme-like proteins on the ends of their tails. These proteins penetrate the periplasm to digest the peptidoglycan barrier.
Self-assembly pathway of phages
- Multiple copies of the capsid or scaffold complex bind the portal protein to form the procapsid
- The scaffold proteins are ejected
- DNA is packaged into the procapsid
- This procapsid then expands to the size of the mature capsid
- The head completion proteins are then bound to the portal complex to prevent DNA leakage.
- Decoration proteins bind to the capsid
- The tail that is assembled separately or after DNA packaging is then attached to the capsid
- Then, the final infectious phage is produced
- Note: It is the preassembled tail that attaches to the capsid in Myoviridae and Siphoviridae. Then, in Podoviridae the tail assembles on the capsids at the stopper
Bacteriophage life cycle
Bacteriophages like every other virus must infect and hijack a host cell to be able to reproduce. Hence, the steps that form this infection process are referred to as the lifecycle of the bacteriophage.
Some bacteriophages can only reproduce when they burst and kill their host cells whereas some may not kill the host cell and are replicated each time the host cell divides. However, there are basically 2 different cycles at which bacterial viruses employ to infect bacteria. These life cycles are:
- Lytic cycle (virulent)
- Lysogenic cycle (temperate)
Lytic vs Lysogenic cycle
During infection, a bacteriophage attaches to its host and inserts its genome into the cell. After this, the bacteriophage usually follows either a lytic or lysogenic cycle. The phages that follow the lytic cycle hijack the cell of the host to make phage components. Then, they release new bacteriophages as they lyse or destroy the cell.
The bacteriophages that follow the lysogenic cycle introduce their nucleic acid into the chromosome of the host cell. Then, they replicate with the cell as a unit without destroying it. However, under certain conditions, lysogenic phages can be enhanced to follow a lytic cycle.
-
Lytic cycle
In the lytic cycle, the phage infects the bacterium and hijacks the host cell. It uses the cell’s resources to produce many new bacteriophages. This causes the host cell to burst (lyse) and die. Bacteriophages that undergo the lytic cycle are called virulent phages.
Phases in the lytic cycle:
- Attachment: The protein in the tail of the bacteriophage attaches to a specific receptor on the bacterial cell’s surface.
- Entry: The bacteriophage then injects its dsDNA genome into the bacterium’s cytoplasm.
- DNA copying and protein synthesis: The DNA of the bacteriophage is copied and the genes of the bacteriophage are then expressed to make proteins like capsid proteins.
- Assembly of new bacteriophages: The capsids assemble from the capsid proteins. Then, the capsid is stuffed with DNA to make many new bacteriophage particles.
- Lysis: Lastly, in the lytic cycle, the bacteriophage expresses genes for proteins that make holes in the cell wall and plasma membrane of the host. These holes allow water to flow in. Hence resulting in the host cell expanding and exploding. Lysis releases hundreds of new bacteriophages that search and infect other host cells. Hence, a few cycles of lytic infection can encourage the widespread of bacteriophages through a bacterial population.
The T4 phage for example has a lytic cycle. The bacterial cells are lysed by the t4 phage and destroyed after immediate replication of the virion. Immediately the cell is destroyed, the bacteriophage progeny finds new hosts to infect.
From research, it is seen that lytic phages are more suitable for phage therapy. Also, a phenomenon known as lysis inhibition is a process that some lytic phages go through. In this condition, the completed phage progeny will not completely burst out of the cell if extracellular bacteriophage concentrations are high. This lysis inhibition is usually temporary and is not similar to lysogenic phages going dormant.
-
Lysogenic cycle
In the lysogenic cycle, the phage infects a bacterium and injects its DNA into the chromosome of the bacterium. This allows the DNA of the bacteriophage (prophage) to be copied and passed on together with the DNA of the cell.
This type of life cycle allows a phage to reproduce without killing the host cell. However, some phages can use only the lytic cycle but a phage like the lambda of E. coli can switch between two cycles. This bacteriophage is known to follow the lysogenic cycle and the lytic cycle.
Bacteriophages that undergo the lysogenic cycle (lysogeny) are called temperate phages. The viral genome of the phage will integrate with the DNA of the host and replicate together with it. This is harmless to the cell. The virus remains dormant until the conditions of the host deteriorate as a result of nutrients depletion. Then, the endogenous phages which are called the prophages, become active. When active, they initiate the reproductive cycle that results in the lysis of the host cell.
The virus is replicated eventually in all daughter cells as the lysogenic cycle allows the host cell to stay alive and reproduce. However, when the prophage is dormant it can provide benefits sometimes to the host bacterium. It adds new functions to the genome of the bacterium in a phenomenon called a lysogenic conversion. An example is bacteriophage converting harmless strains of Corynebacterium dipheriae to virulent ones that cause diphtheria. Another example is bacteriophage converting the harmless strains of Vibrio cholerae into virulent strains that cause cholera.
Phases in the lysogenic cycle:
- Attachment: The phage attaches to the cell of the bacteria.
- Entry: The bacteriophage inserts its DNA into the bacterial cell.
- Integration: The DNA of the bacteriophage then recombines with the chromosome of the bacteria and becomes integrated into the chromosome as a prophage. However, the prophage is not active and doesn’t spearhead the production of new phages.
- Cell division: Every time a cell that contains the prophage divides, the daughter cells inherit the prophage. Once the host cell is divided the prophage is copied together with the DNA of the host. Hence, getting a free exit ride.
In the lysogenic cycle, the first two phases that occur are attachment and DNA injection just like in the lytic cycle. However, different from the lytic cycle, in the lysogenic cycle, once the DNA of the bacteriophage is inside the cell, it is not copied immediately to make proteins. Rather, it binds with a particular part of the bacterial chromosome. Hence, causing the DNA of the bacteriophage to be integrated into the chromosome. Thus, the lysogenic cycle does not lead to the immediate lysing of the host cell.
Also, it is important to note that during the integration phase of the lysogenic cycle, the prophage can become active under the right conditions and come back out of the chromosome of the bacterial cell. This triggers the remaining phases of the lytic cycle that involves DNA copying and protein synthesis, phage assembly, and lysis. The prophage in such a case exits the chromosome and becomes its own circularized DNA molecule. Then, the lytic cycle commences.
How does a phage choose a lytic or lysogenic cycle?
A bacteriophage chooses whether to enter the lytic or lysogenic cycle when it infects a bacterium. A major factor that influences this decision is the number of phages infecting the cell at once. When there are larger numbers of co-infecting phages, it is more likely that the infection will use the lysogenic cycle. This may help prevent the phages from killing their hosts by toning down the infection once the ratio of phage-to-host gets too high.
Additionally, some conditions trigger a prophage to pop back out of the chromosome and enter the lytic cycle. Some DNA-damaging agents such as UV radiation and chemicals for instance, in the laboratory, will trigger most prophages in a population to re-activate and go lytic. Though, a little fraction of the prophages in a population spontaneously choose to go lytic even without these external cues.
Other life cycles of bacteriophages
There are other life cycles that bacteriophage use, such as pseudolysogeny and chronic infection. In pseudolysogeny, the phage enters a cell but doesn’t adopt a cell-replication system or integrates stably into the genome of the host. However, pseudolysogeny happens when a host cell encounters unfavorable growth conditions. This type of life cycle seems to play an important role in the survival of the phage by allowing the preservation of the phage genome until host growth conditions have become favorable again. In chronic infection, new phage components are produced without killing the cell. Apparently, in this cycle, new phage components are generated over a long period of time, continuously, without killing the cell.
Types of bacteriophage
- Lytic or virulent bacteriophage
- Temperate or lysogenic bacteriophage
There are two main types of bacteriophages, which are lytic bacteriophages and temperate bacteriophages. Lytic bacteriophages are phages that reproduce through the lytic life cycle and as part of their life cycle lyse the host bacterium. Examples of virulent phages are the Pseudomonas virus phi6, T-phages: T1 phage, T2 phage, T3 phage, T4 phage, T5 phage. T6 phage, T7 phage.
Temperate bacteriophages are phages that are capable of a lysogenic life cycle. The temperate phage either replicate by means of the lytic life cycle or incorporate its DNA into the bacterium’s DNA and become a prophage. The lambda phage is an example of temperate bacteriophages. Others include the P1 phage.
Bacteriophage examples
- Lambda phage
- t4 bacteriophage
- Enterobacteria phage T2
- Bacteriophage MS2
- Enterobacteria phage T4
- Pseudomonas virus phi6
- Enterobacteria phage T6
- P1 phage
- Escherichia virus T5
- Bacillus virus phi29
- Ligamenvirales
- Ampullaviridae
- Phi X 174
- Plasmaviridae
- Lipothrixviridae
- Fuselloviridae
- Bacteriophage AP205
- Clavaviridae
- Globuloviridae
- Guttaviridae
- Bicaudaviridae
- M13 bacteriophage
-
Lambda phage
Lambda phage also called Enterobacteria phage λ or Escherichia virus Lambda infects the bacterial species- Escherichia coli (E. coli). In 1950, the lambda phage was discovered by Esther Lederberg. The wild variant of the lambda virus has a temperate life cycle. This allows it to either be lysogenic by living within the genome of its host or enter into a lytic phase in which it lyses the cell to produce offspring.
The capsid of the lambda has the phage’s double-strand linear DNA genome. During the infection process, the phage particle recognizes and binds to the host E. coli. This causes the DNA in the capsid of the phage to be ejected through the tail into the cytoplasm of the E.coli cell.
The lambda DNA is then replicated and new phage components are produced within the cell. Cell lysis, however, follows this, where the cell contents that have been assembled are released into the environment. Though, under certain conditions, the lambda phage DNA may integrate itself into the chromosome of the host cell. Hence, following the lysogenic pathway. In this state, the lambda DNA becomes a prophage and lives within the genome of the E.coli without harming the host. The host E.coli however, is called a lysogen when a prophage is present. Moreso, when the lysogen enters a stressed condition, the prophage may enter the lytic cycle.
-
T4 bacteriophage
Escherichia virus T4 commonly called the T4 bacteriophage is a species of bacteriophages that infect E. coli bacteria. T4 is a double-stranded DNA virus from the family Myoviridae. It is capable of only using a lytic lifecycle and not the temperate lifecycle. These viruses were formerly called T-even bacteriophages. This name also covers Enterobacteria phage T2, Enterobacteria phage T4, and Enterobacteria phage T6.
Phage T4’s complete genome sequence consists of 168,903 base pairs. It encodes about 300 gene products. T4 bacteriophages are virulent and play a major role in virology and molecular biology.
How does the T4 infect its host? The virus infects Escherichia coli by binding on the surface of E. coli cells using its long tail fibers. Through the long tail fibers, a recognition signal is sent to the baseplate. This clears up the short tail fibers (STF) that irreversibly bind to the cell surface of the E. coli cell. The tail sheath contracts as the baseplate change conformation.
This causes the glycoprotein V (GP5) at the end of the tail tube to puncture the cell’s outer membrane. Then, the lysozyme domain of the glycoprotein V becomes active and degrades the periplasmic peptidoglycan layer of the host. However, the part of the membrane remaining is degraded. Then, DNA from the capsid travels through the tail tube to the cell of the E.coli.
-
Enterobacteria phage T2
The enterobacteria phage T2 belongs to the family Myoviridae and infects and kills E.coli. It is a linear double-stranded DNA virus with repeats at either end. This virus is covered by a protective protein coat.
The T2 phage is lytic as it can turn an E.coli cell into a T2-producing system that releases T2 phages when the cell bursts. T2 is one of the first bacteriophages that were studied in detail. They were seven of them that commonly infect E.coli i.e T1-T7. However, T2, T4, and T6 phages are now called T-even phages as a result of the similarities in their structure.
The T2 bacteriophage using the proteins on its tail fibers attaches to the surface of an E.coli bacterium. Then, it introduces its genetic material (either DNA or RNA). The genome, however, makes use of the host cell’s ribosomes to replicate. It also synthesizes proteins for the phage capsid and tail. Then, new T2 phages are assembled within the cell until the cellular membrane burst open. The newly produced T2 phages are then free to move in search of more cells to infect. Hence, the T2 phage uses the lytic cycle.
-
Bacteriophage MS2
Bacteriophage MS2 is commonly called MS2. This phage is an icosahedral, positive-sense single-stranded RNA virus. The MS2 infects Escherichia coli and other members of the Enterobacteriaceae. MS2 belongs to the same family of phages like bacteriophage f2, bacteriophage Qβ, R17, and GA.
MS2 usually infects enteric bacteria by carrying the fertility (F) factor. The fertility factor is a plasmid that allows cells to act as DNA donors in bacterial conjugation. However, genes on the plasmid lead to the production of an F pilus. This F pilus acts as the viral receptor. The bacteriophage MS2 through its single maturation protein attaches to the side of the pilus. However, the exact mechanism at which the RNA of the phage enters the bacterium is unknown.
-
Pseudomonas virus phi6
Pseudomonas virus Phi6 happens to be the best-studied bacteriophage. It belongs to the virus family Cystoviridae. This phage infects Pseudomonas bacteria, most especially the plant-pathogenic species- Pseudomonas syringae. Phi6 has a three-part segmented double-stranded RNA genome. This virus has a lipid membrane around its nucleocapsid which is a rare trait among other bacteriophages. It is a virulent bacteriophage even though under certain conditions has been observed to display a delay in lysis. This act may be described as a carrier state.
-
P1 phage
P1 phage infects Escherichia coli as well as some other bacteria. This virus is a temperate bacteriophage. The genome of this phage exists as a plasmid when undergoing a lysogenic cycle, unlike other phages. Other phages like the lambda phage usually integrate into the DNA of the host.
P1 phage has an icosahedral head that contains the DNA. This capsid head is attached to a contractile tail that has six tail fibers. This bacteriophage can be used in transferring DNA from one bacterial cell to another. Due to this process of transduction, it has gained research interest. As the virus replicates during its lytic cycle it grabs fragments of the host chromosome.
However, if the viral particles that result from this are used to infect a different host, the DNA fragments that were grabbed can be integrated into the new host’s genome. For many years, this method of in vivo genetic engineering was widely used even today. Though, it is to a lesser extent that this method is used.
The P1 phage is also used to form the P1-derived artificial chromosome cloning vector. This vector can carry large fragments of DNA. P1 phage is also involved in Cre-Lox recombination.
-
Escherichia virus T5
Escherichia virus T5 is also called Bacteriophage T5. This is a caudal virus that belongs to the family Demerecviridae. This phage follows a lytic cycle and specifically infects E.coli bacterial cells.
Bacteriophage T5 infects E.coli by using its receptor-binding protein to bind to the outer membrane ferrichrome transporter (FhuA) of the host cell. This bond triggers structural changes in the receptor-binding protein and leads to the phage releasing DNA from the capsid.
-
Bacillus virus phi29
Bacillus virus phi29 is a phage that belongs to the order Caudovirales. Other phages in this order are phages PZA, Φ15, BS32, B103, M2Y (M2), Nf, and GA-1. They are the smallest Bacillus phages and are among the smallest double-stranded DNA phages.
Ligamenvirales are linear bacterial viruses that infect archaea. They are double-stranded DNA viruses. Their name, however, is derived from the Latin word ligamen which means string or thread.
-
Ampullaviridae
Ampullaviridae is a family of viruses. They infect archaea that are of the genus Acidianus. The viruses in this family have the shape of a bottle. Hence, the family and genus name of the virus was derived from the Latin word ampulla which means bottle.
The replication of these viruses is cytoplasmic. In order to enter the host cell, the virus attaches to the host cell. The method of transcription is DNA-templated transcription and the transmission routes are passive diffusion.
-
Phi X 174
The phi X 174 is a single-stranded DNA (ssDNA) bacteriophage. This virus attack and infects Escherichia coli. Also, it is the 1st DNA-based genome to be sequenced. Arthur Kornberg used this virus as a model to first prove that DNA synthesized in a test tube by purified enzymes could generate all the characteristics of a natural virus.
-
Plasmaviridae
Plasmaviridae is actually a family of bacterial viruses. The natural host to these viruses is the Acholeplasma species. However, this virus family is poorly studied. Hence, little is known about the biology and diversity of the virus. An infectious cycle begins prior to a lysogenic cycle in the infected bacteria. The virus, however, may become latent within the host after the initial infection of the genome.
-
Fuselloviridae
Fuselloviridae is a virus family. There are 9 species of virus and 2 genera in the family. Phages belonging to the Fuselloviridae are ubiquitous in high-temperature and acidic in hot springs.
Replication of the virus is cytoplasmic. They enter the host cell by adsorption into the host cell. The method of transcription is DNA templated transcription. Also, their natural hosts are Sulfolobus shibatae, Sulfolobus solfataricus, and Sulfolobus islandicus. Fuselloviruses are released from the host by a budding mechanism. They do not cause cell lysis. Moreso, the budding mechanism they employ is similar to that used by the enveloped eukaryotic viruses.
-
Clavaviridae
Clavaviridae is a family of phages. These bacteriophages are double-stranded DNA viruses that infect archaea. Clavavirus is the only genus in this family. The family name was derived from the Latin word clava which means stick.
These viruses are bacilliform in shape with one end pointed and the other rounded. They do not integrate into the genome of the host and don’t cause the host cell to lyse.
-
Guttaviridae
Guttaviridae is another family of viruses that infect archaea. There are 2 genera in this family that contain a species each. The family name is derived from the Latin word gutta which means droplet. The method of transcription is DNA-templated transcription.
-
Bicaudaviridae
Bicaudaviridae is a family of viruses. They are hyperthermophilic archaeal viruses. Archaea of the genus Acidianus are the natural hosts of these viruses. There is only one genus and 1 species in this family. Replication of the virus is cytoplasmic and the virus enters the host by attaching its viral proteins to the host receptors. The method of transcription is DNA-templated transcription.
Transmission is via passive diffusion. It has been shown that certain viruses of this family induce cell gigantism. Examples are the Single tailed Sulfolobus virus (STSV2) and Sulfolobus monocaudavirus 1 (SMV1). They block the expression of the cell division genes and arrest the cell cycle in the S phase. As a result, the diameter of the infected cells increases up to 20 times. This leads to an 8000-fold increase in volume compared to cells that are not infected.
-
M13 bacteriophage
M13 bacteriophage is one of the Ff phages. The Ff phages is a group of almost identical filamentous bacteriophages which are composed of circular single-stranded DNA. Other Ff phages are fd and f1.
The minor coat protein (p3) of M13 attaches to the receptor that is at the tip of the host Escherichia coli F pilus. This phage has a relatively short life cycle. Ten minutes after infection, the early phage progeny exits the cell.
These phages are chronic phages. Hence, they release their progeny without destroying the host cells. However, compared to the regular lysis, their infection causes turbid plaques in E.coli lawns. Moreso, a reduction in the cell growth rate is seen in cells that are infected. Furthermore, M13 plasmids are used for several recombinant DNA processes. Also, M13 has been used for directed evolution, phage display, nanostructures, and nanotechnology applications.
Phage therapy
Phage therapy (PT) involves the use of phages or bacterial viruses to treat bacterial infection. It is also called bacteriophage therapy. Bacteriophages only attack bacteria and are harmless to people, animals, and plants. Hence, they are used for this therapy.
Phages are the natural enemies of bacteria and are found in soil, sewage, water, and other places that bacteria live. Hence, they naturally help to keep bacteria growth in check. Phage therapy isn’t a new thing and has been used for years. Though the treatment isn’t well known and more research on bacteriophages is required. However, phage therapy may be a useful alternative to antibiotics for disease-causing bacteria.
How does phage therapy work?
Phage therapy works because bacteriophages kill bacteria by making them lyse or burst. This occurs when the virus binds to the bacteria and infects it by injecting its genome (DNA or RNA) into the bacteria. The bacteriophage then reproduces and replicates itself inside the bacteria. This can result in thousands of new bacteriophages in each bacterium. Then, the bacteria eventually burst open releasing the new bacteriophages. Once the bacteria are lysed and dead, the bacteriophages stop multiplying. This is because they only grow and multiply inside a bacterium. Also, the phages may lay dormant in a sort of hibernation state till more bacteria shows up.
Advantages of phage therapy
- There are many kinds of bacteria and each kind of phage only attacks a certain bacterium. This means that a bacteriophage can be used to target and kill disease-causing bacteria. For instance, a strep phage will only kill bacteria that cause strep throat infections.
- Bacteriophages work against treatable and antibiotic-resistant bacteria.
- Phages can be used alone or with antibiotics and other drugs.
- Only one dose may be needed during treatment as phages multiply and increase in number by themselves.
- Phages only slightly disturb the normal helpful bacteria in the body.
- Bacterial viruses are natural and easy to find.
- Phages are not harmful (toxic) to the human body neither are they harmful to animals, plants, and the environment.
Phage therapy disadvantages
- Phage therapy needs more research and as a result, phages are not widely used yet. It’s not well known if these phages may harm humans or animals in ways other than direct toxicity.
- It is also not well known if the bacteriophage therapy may cause bacteria to become stronger than the phage used. This can result in phage resistance as the bacteria may likely become resistant.
- Currently, phages are difficult to prepare for use in humans and animals.
- Research on what dose or amount of phages to be used and how long the phage therapy may take to work is still not known.
- Finding the exact bacteriophage required to treat an infection may be difficult.
- Phages may probably cause the immune system to overreact or probably cause an imbalance.
- Some types of bacteriophages don’t work like other phages in treating bacterial infections. Hence, there may not be enough kinds of phages to treat all bacterial infections.
- Furthermore, phages of Inoviridae have been shown to complicate the biofilms involved in cystic fibrosis and pneumonia. Also, they protect the bacteria from drugs that are meant to eradicate disease, hence promoting persistent infection.