Table of Contents
What is a biogeochemical cycle?
A biogeochemical cycle is a pathway in ecology and Earth science by which a chemical substance is turned over or moves through the biosphere and Earth’s lithosphere, atmosphere, and hydrosphere.
A biogeochemical cycle involves the exchanges of chemical elements between different parts of the Earth, such as the atmosphere, land, and sea, soils, and plants. It is called a cycle because matter (materials), elements move to and from huge reservoirs via a variety of a two-way flow system.
This is despite the fact that some elements are preserved in places or forms that are selectively accessible to living things. For example, nitrogen in the atmosphere needs to undergo a series of conversions before it can be assimilated and used by plants in the soil (lithosphere).
Definition of biogeochemical cycle
A biogeochemical cycle is among the natural cycles that move stored matter through an ecosystem’s biotic and abiotic components. The stored matter can be oxygen, nitrogen, sulfur, phosphorus, and carbon.
These elements are very essential to the living cells of both plants and animals to function properly. Their significance or importance means that they need to cycle through the different spheres of the Earth in order to maintain a balance of the element in all these spheres. A balance is indeed necessary to avoid a backlash of the element on the earth, in the form of a deficiency of any of the elements in any of the spheres hence, the need for the cycle of these elements.
Biogeochemical worksheet
Questions |
Answers |
What is a biogeochemical cycle? |
|
List some examples of a biogeochemical cycle? |
|
What are the steps in the carbon cycle? |
|
How many steps are there in the oxygen cycle? |
|
What is denitrification? |
|
Summarize the sulfur cycle |
|
Explain decomposition as a step in the phosphorous cycle |
|
What is transpiration? |
|
What is the role of decomposers in the carbon cycle? |
The above is an example of a typical biogeochemical worksheet that one can use to teach biogeochemical cycles or utilized as a study guide to simply understand biogeochemical cycles.
Examples of biogeochemical cycle
- Carbon cycle
- Water cycle
- Nitrogen cycle
- Oxygen cycle
- Phosphorus cycle
- Sulfur cycle
The above listed are some examples of a biogeochemical cycle, and these examples are briefly explained below.
Carbon cycle
Carbon is present in all living things, and many other non-living things like minerals, the atmosphere, oceans, and the earth’s interior. Because it is a key component of biological compounds, it is indeed critical in maintaining a balance between the amount of carbon stored in sinks and the amount released from various sources in order to preserve life.
Carbon movement through the land, water, and the air is sophisticated because carbon is preserved in carbon reservoirs, such as the atmosphere, bodies of liquid water (mostly oceans), ocean sediment, soil, land sediments, and the earth’s interior. The atmosphere is a major reservoir of carbon in the form of carbon dioxide, which is critical to the photosynthesis process.
The carbon reservoir in the oceans has a significant impact on the level of carbon dioxide in the atmosphere because the interchange of carbon between the atmosphere and water reservoirs influences how much carbon is found in each location. This means that each has a reciprocal effect on the other.
Steps in the Carbon Cycle
- Carbon Intake into the Atmosphere
- Producers’ Absorption of Carbon Dioxide
- Carbon Compounds Passing Through the Food Chain
- Carbon Return to the Atmosphere
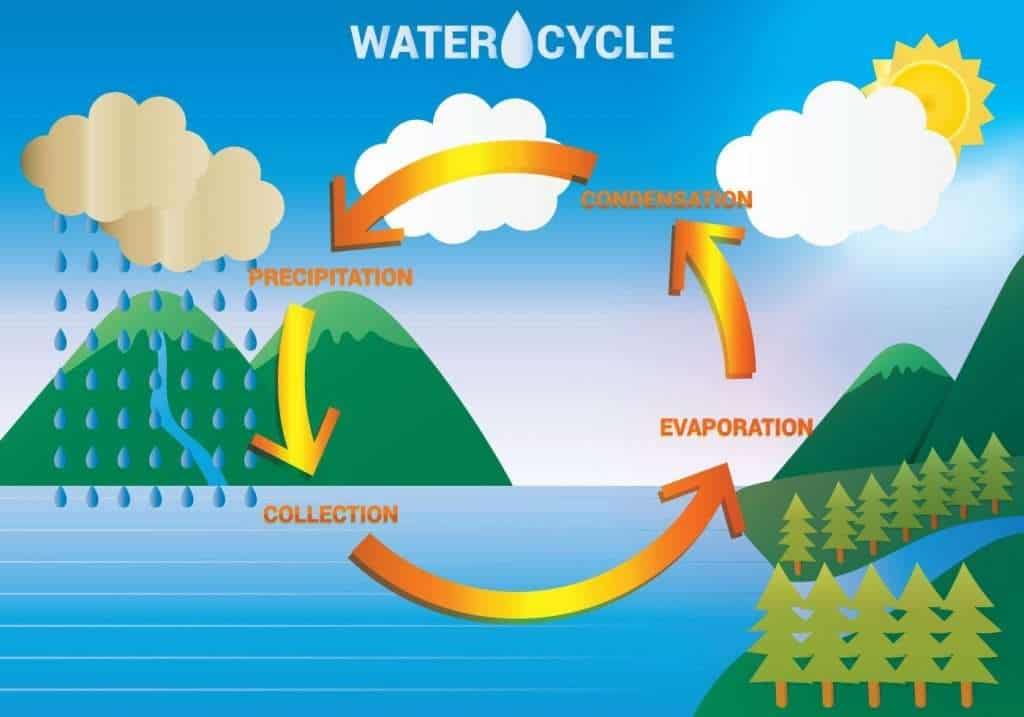
Water cycle or hydrological cycle
The hydrological cycle, also known as the biogeochemical cycle of water, describes how water (H2O) is distributed or circulated, and recycled all across Earth’s systems.
Water is essential for all living organisms to survive and grow, making it one of the most essential substances on the planet. It is utilized by complex organisms to dissolve vitamins and minerals and to transport these substances (minerals and vitamins), along with hormones, antibodies, oxygen, and other substances, throughout the body and out of the body in the form of sweat. It also supports the enzymatic and chemical reactions needed for metabolic activities and to regulate body temperature.
Weather patterns are caused by the biogeochemical cycle of water on a geographical scale, especially in the temperature, amount, and movement of the water which all have an impact on all weather systems. Water interacts with its surroundings in various forms (vapor, liquid, and ice), and in doing so, it changes the temperature and pressure of the atmosphere, causing wind, rain, and currents, and changing the structure of earth and rock through weathering.
The water cycle steps
- Evaporation
- Sublimation
- Transpiration
- Condensation
- Precipitation
- Runoff/snowmelt
- Infiltration
The sun’s energy, which heats up the oceans and other surface waters, is the driving force of the water cycle. This is because the heat causes evaporation (the conversion of liquid surface water to water vapor) and sublimation (the conversion of ice to water vapor) of frozen water, both of which deposit large amounts of water vapor into the atmosphere.
This water vapor condenses into clouds as a liquid or frozen droplets over time, which is then preceded by precipitation (rain or snow), which returns water to the earth’s surface. The rain finally filters down into the ground, where it may evaporate again (if near the surface), or flow beneath the surface, or be stored for extended periods of time inside the lithosphere as part of the groundwater.
Surface runoff (the flow of freshwater from rain or melting ice) is more visible and can make its way to the oceans via streams and lakes, or it can flow directly to the oceans. Rain and surface runoff are major carriers of minerals such as carbon, nitrogen, phosphorus, and sulfur from land to water. This simply shows how one biogeochemical cycle can help other biogeochemical cycles to complete their cycles.
Nitrogen cycle
The nitrogen cycle is a continuous cycle of nitrogen, in which nitrogen flows through both living and non-living things, including the atmosphere, soil, water, plants, animals, and bacteria (which are microscopic living organisms with only one cell that can be found everywhere). Nitrogen must change forms in order to move through the various stages of the cycle. Nitrogen enters the water via rainfall and snow, runoff, or atmospheric N2.
Nitrogen cannot be used as N2 by phytoplankton, so it must experience nitrogen fixation, which is primarily accomplished by cyanobacteria.
The nitrogen cycle is also an essential process in the ocean and the overall cycle is similar to that of the cycle in the atmosphere and the lithosphere, different players and mechanisms of nitrogen transfer exist in the ocean.
Steps of the nitrogen cycle
- Nitrogen fixation
- Mineralization
- Nitrification
- Immobilization
- Denitrification
Oxygen cycle
The oxygen cycle is critical to the survival of life on Earth. It is a biological process that helps to maintain the oxygen level by moving through the earth’s three major spheres, namely:
- Atmosphere
- Lithosphere
- Biosphere
The oxygen cycle explains how oxygen gas travels through the atmosphere, ecosystem, biosphere, and lithosphere. The oxygen cycle is simultaneously linked to the carbon cycle.
Steps of the oxygen cycle
- As a byproduct of photosynthesis, all green plants release oxygen back into the atmosphere.
- All aerobic organisms respire using free oxygen.
- Animals release carbon dioxide into the atmosphere, which plants use again during photosynthesis. The amount of oxygen in the atmosphere is now balanced.
Phosphorus cycle
Phosphorus is a necessary nutrient for all living processes. For example, it is a crucial constituent of nucleic acid, both DNA and RNA; phospholipids, (which are the main component of cell membranes); and, calcium phosphate, (which is a component of our bones’ supportive components). In aquatic ecosystems, phosphorus is frequently the key nutrient (necessary for growth).
Phosphorus exists as the phosphate ion caused by human activity which is a factor for the availability of phosphorus in aquatic systems. Human activities are not the only factor, the leaching of phosphate-containing rock by weathering, causes phosphates to enter rivers, lakes, and the ocean. Phosphorus is also exchanged back and forth between phosphate dissolved in the ocean and marine ecosystems. The motion of phosphate from the ocean to the land and through the soil is incredibly slow, with the average phosphate ion spending twenty thousand to one hundred thousand years in the ocean.
The phosphorus cycle differs from the other major biogeochemical cycles in that it lacks a gas phase; however, small amounts of phosphoric acid (H3PO4) may enter the atmosphere, contributing to acid rain in some cases.
Steps of the phosphorus cycle
- Weathering/ human activity.
- Plant absorption.
- Animal absorption.
- Decomposition leading to its return to the environment.
Sulfur cycle
Sulfur is a necessary component of the living things’ macromolecules. It is involved in the formation of disulfide bonds within proteins as part of the amino acid cysteine, which helps to determine their 3-D folding patterns and, their functions. Sulfur cycles exist in the oceans, on land, and in the atmosphere.
Precipitation, direct fallout from the atmosphere, rock weathering, and organic material decomposition by decomposers are the 4 ways in which sulfur is deposited on land.
Sulfur dioxide is the most common form of sulfur in the atmosphere. As rain falls through the atmosphere, sulfur dissolves as weak sulfuric acid (H2SO4), resulting in acid rain. Sulfur can also fall directly from the atmosphere as a result of a process known as fallout. Weathering of sulfur-containing rocks releases sulfur into the soil as well.
Sulfur is released back into the atmosphere as hydrogen sulfide (H2S) gas after these organisms die and decompose. Geothermal vents can also release sulfur into the atmosphere.
Sulfur enters the ocean through land runoff, fallout, and underwater geothermal vents. Chemoautotrophs, which use sulfur as a biological energy source, is important in some marine ecosystems.
Sulfur cycle steps
- Organic compound decomposition
- Hydrogen sulfide oxidation to elemental Sulphur
- Elemental sulfur oxidation
- Sulfate reduction
Importance of biogeochemical cycle
- A biogeochemical cycle assists in explaining how the planet conserves matter and uses energy.
- Function as a transport system for elements through ecosystems. This cycle allows for the transformation of elements through the cycles.
- It is also important because it stores and recycles elements.
- A biogeochemical cycle can demonstrate the interdependence of all living and nonliving things on Earth.